Today is Clinical Trials Day, organized by the Association for Clinical Research Professionals. Clinical Trials Day is a day for celebration and recognition of clinical trial professionals and the work they do every day to improve patient lives and bring new treatments to market.
New interventions are a source of hope for many patients in need, and a culmination of years and decades of hard work from dedicated clinical development professionals who relentlessly strive to make clinical trials more successful. We recognize and celebrate the hard work done by clinical research professionals today and every day.
Today is also a moment for reflection. While there have been significant improvements in how clinical trials are operated, there are still a number of challenges remaining for both clinical trial professionals and trial participants. These range from recruitment and retention, to design and implementation, to safety and efficacy issues, which ultimately lead less than 15% of clinical trials to successful drug approval.
Moreover, even a successful trial completion does not tell the full story. Long-term follow up is needed for Real World Evidence – to demonstrate efficacy signals and provide peace of mind around an intervention’s safety. While solving these challenges requires multiple approaches, we recognize that a common denominator across these is a data connectivity challenge.
As we celebrate clinical development, the industry should think about the next generation of clinical trials and how connected clinical trials, which link trial data to real-world data, can improve clinical development success rates and propel innovation.
At Datavant, our mission is to connect the world’s health data to improve patient outcomes. We have recognized that the fragmentation of healthcare data is a central problem to improving those outcomes, far more than data availability. This is especially true in clinical development, where trials are run as their own (very strategic, but very expensive!) data silo.
However, a patient’s journey is far more encompassing than the time spent in a clinical trial. It involves their medical history before the trial, their time on an intervention after the trial is complete, and various choices made about their health, even outside of their interactions with healthcare professionals. There is immense wealth in that information, and that information can elucidate important insights during a clinical trial.
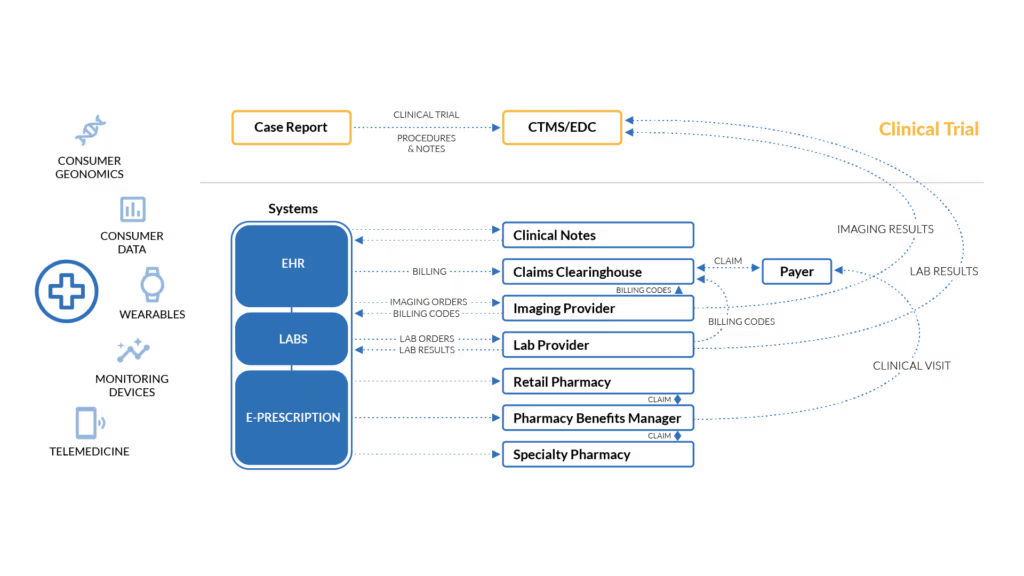
Figure 1. The problem: A trial participant’s journey involves multiple, fragmented encounters which generate health data, including data generated as part of a clinical trial.
Connected clinical trials, where clinical data links to a participant’s information in the real world can truly improve patient outcomes. In 2019, we introduced Datavant for Clinical Trials solutions to power that data linkage.

Figure 2. The solution: Connecting clinical trial data to a participant’s data outside of a clinical trial can help answer specific questions and improve clinical outcomes.
We believe a next generation clinical trial requires the following to be successful:
- A privacy-preserving way to connect clinical trial and real-world data (we call this Trial Tokenization)
- Timely, complete, fit for purpose, real-world data that can help ask and answer the right questions
- Collaboration across the clinical trial ecosystem (sponsors, technology and service providers, clinical professionals, academics, regulatory and ethics groups, advocacy groups, and the trial participants themselves) to define and implement best practices so that every trial can be connected
Over the next few months, we will be sharing best practices around how the next generation of connected clinical trials can help better answer questions and resolve clinical trial challenges, as well as some use cases that our partners and customers have unlocked by bridging clinical trial and real-world data. We look forward to continuing the conversation!
Datavant is keen to collaborate with anyone who is interested in creating the next generation of clinical trials. Please visit our Clinical Development Resources page or contact us at info@datavant.com if you want to learn more and collaborate with us.
Editor’s note: This post has been updated on December 2022 for accuracy and comprehensiveness.





.svg)










